feature
Advancing Alloys
Bringing solid mixtures to the high school classroom
The Science Teacher—March 2020 (Volume 87, Issue 7)
By Christina Polcino, Billyjack Jory, Jean Sabety, Laura Grenot Jones, Jared Ashcroft, and Brandon Rodriguez

The manufacture of metal alloys is ubiquitous, yet infrequently discussed in high school coursework as concepts related to them are often too complex or abstract for beginning science students. However, earlier introduction to metallurgy in classroom settings could promote interest in practical applications of chemistry, physics, and geology due to the demonstrable significance of materials in daily use. Elemental metals with low melting points such as bismuth, zinc and tin are melted down, mixed at various percent compositions, and allowed to solidify. The solidified composites are then broken open to examine resulting internal structures. Changes in physical properties of new compositions are observed, analyzed, and discussed.
Experimental results are the basis for a multi-tiered laboratory experiment for high school chemistry or geoscience courses. From introductory, inquiry-based techniques in chemistry to more advanced concepts in petrology, each lab can be tuned to cover a wide spectrum of multi-dimensional science applications.
Demand for cross-curricular activities with real-world applications is higher than ever with the Next Generation Science Standards (NGSS 2013) being implemented more widely. We developed an inquiry-based lab that teaches students about alloy creation—a thousand-year-old practice that is the basis of our modern infrastructure—while also introducing interdisciplinary aspects of chemistry and geology.
This alloy lab bridges classroom learning with scientific methods to teach students about chemistry, physics, and geology, while simultaneously promoting scientific discovery and interest. We implemented the activity using the BSCS 5E Instructional Model, an inquiry-based approach in which students learn new topics based on existing knowledge (Bybee 2016). The 5E Model is particularly suited to this lab due to high school students’ general unfamiliarity with alloys and their applications, and it allows for a complete learning cycle in which students are engaged at every step of the learning process.
Engage
In 1998, the same year Microsoft unveiled its iconic new operating system, a new age of space exploration began with the first component of the International Space Station (ISS; Figure 1) being launched into orbit. By the year 2000, ISS’s first long-term residents arrived, and it has since served as a valuable research laboratory for a multitude of disciplines, as well as a testing environment for spaceflight systems and equipment required for missions to the Moon, Mars, and beyond.
Among the hundreds of materials required to manufacture the ISS and other spacefaring vehicles, aluminum alloy is one of the most common (see “On the web”). Composed primarily of aluminum and copper (Holt and Ho 1996), this lightweight alloy’s high strength and ability to withstand a temperature range of -268°C to 315°C (-452°F to 600°F) has assisted in making the ISS a monumental human achievement.
Back on Earth, humans have been fashioning metal alloys since 3000 BC, and modern life would simply not exist without them. High-quality alloys for modern applications are in high demand, but alloys are rarely mentioned in science classrooms; typically they are only introduced as “a mixture of two solids” before returning to solution chemistry. While concepts surrounding metal alloys, such as eutectics and metallurgy, are generally reserved for upper division coursework due to their complexity, a basic understanding of the ubiquity and preparation of alloys can be achieved with beginning science students.
The potential for this lab to be fine-tuned based on the academic level of students, combined with the relatively low cost of materials (and availability of a standard chemistry classroom), make the experiment accessible to a wide variety of educational institutions. We successfully taught the lab to both junior and senior students at a Title I high school, as well as in an introductory chemistry course at a community college. The high school population consists of 99.7% free and reduced lunch students who are one or more years behind in math and language arts; the community college has a similar student population.
Glossary
| Glossary | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Safety Notes
- Wear safety goggles, apron, and appropriate gloves during the setup, hands-on, and takedown segments of the activity.
- Tie back long hair, secure loose clothing, remove loose jewelry, and remove or cover acrylic nails before starting this activity.
- Use caution when working with Bunsen burners. This heat source can seriously burn skin and clothing.
- Never touch or spill metals in liquid state! They can seriously burn skin.
- Follow the teacher’s directions for disposing of all waste materials at the end of the activity.
- Use caution when working with glassware. It can shatter and cut or puncture skin.
- Activities involving the heating of bismuth and other metals at high temperatures in which noxious or poisonous gases or vapors are produced must be carried out in the fume hood.
- Immediately wipe up any spilled liquid on the floor— slip/fall hazard.
- Use caution when using sharp tools/materials, which can cut or puncture skin.
- Wash hands with soap and water immediately after completing this activity.
Explore
The purpose of this lab is to explore alloy creation for an oft-overlooked study of physical mixtures in chemistry classrooms. The activity centers on identification of unknown alloy composites, composed of two metals. Students begin by observing and then creating binary alloys of bismuth with easily accessible metals. Lower-melting-point metals, such as bismuth, can be crystallized easily and produce a stunning visual display of crystal structures.
Setting the stage for exploration can be achieved by modeling the experiment using pure metals. Teachers can demonstrate investigative techniques with metals such as pure tin, zinc, and bismuth by describing luster, malleability, and other characteristics.
Once expectations are clear, students can break into groups of two to four, and as a class compare observations using a pure metal sample and a teacher-prepared, unknown alloy sample. Students will then create their own alloys of known composition and record their findings to uncover the nature of the unknown alloy composition by comparison.
This lab can be done in three traditional one-hour periods or two block periods. If students are not confident calculating density, the lab can be broken into two parts: first a lesson on alloy creation, then a second on density.
Educators may provide a narrative prior to experimentation similar to the following: You are a materials quality assurance analyst for an aerospace engineering firm and have been given a metal sample to analyze. The firm is working on the creation of a new composite material that could potentially be used in the manufacturing of habitats for an upcoming extended mission to Mars (see Figure 2). The firm wants an alloy of bismuth and tin, of which the bismuth percentage falls between 20% and 40% of total composition. You need to determine if the sample you were given is, in fact, comprised of bismuth and tin, and if so, does it fall within the specified range?
Students will follow the procedure outlined below. After they have discovered whether the sample they created matches the unknown sample, students determine if it is the correct composition of metals and if it falls within the range of percentages given (Figure 3). After lab completion, the educator can lead a guided discussion to answer post-lab questions.
Materials
- ceramic crucibles
- Bunsen burners
- ring stands
- ceramic triangles or mesh squares
- aluminum shot, mossy zinc, mossy tin, and bismuth (available for purchase online)
- glass stir rods
- water bath
- rock hammer
- chisel
- heat-protectant gloves
- non-latex apron
- indirectly vented, chemical-splash goggles
- graduated cylinder
- top load balance
Teacher preparation
To create pure samples of aluminum, tin, zinc, and bismuth: Place a glazed ceramic crucible over a Bunsen burner. Add 30–50 g of aluminum shot to the crucible and let it melt fully, about 30 minutes. Carefully remove the crucible from heat and allow it to cool at room temperature. When the crucible is cool enough to touch with bare hands, invert it and firmly tap against hard surface — the aluminum should pop out. Repeat with 30–50 g mossy tin, 30–50 g mossy zinc, and 30–50 g bismuth. Crack open each sample using chisel and hammer.
To create the unknown sample (entire class can have same unknown to facilitate collaboration): Choose proportions of metals to use: Good proportions are 70:30, 50:50, 60:40, 90:10 bismuth to metal by weight. Note: As concentration of bismuth decreases, the samples become harder to break open, so samples should be at least 30% bismuth. Note: Avoid making unknown sample with aluminum, as it is difficult to melt fully. However, aluminum should still be offered to students as an option in their experimentation. Weigh out metals needed for each sample. Melt both in same crucible following same method as part 1. Note: An ice bath may be prepared to help cool samples faster.
Leading observations
Have students observe color or tarnish (oxidation), luster, density, and how pure and unknown metals cracked (cleavage) to model investigative behavior. Once safety and lab procedures have been discussed, students determine alloy targets to create, which will help identify unknown sample.
Student instructions
Pre-lab
- Write down observations about the physical properties of pure metal samples: What color are they (oxidation)? How did the sample break (cleavage)? How hard are they? Are they dense? Shiny? How are samples different from each other? How are they similar?
- Look at your unknown sample and make observations. The sample is a mix of bismuth and one other metal, which may be tin, zinc, or aluminum. Based on the pure samples and the observations you have made about them, which metal do you think has been added to bismuth?
- Once your group has decided on the possible metals that make up the sample, choose the composition of the metals. Do samples seem to have more bismuth, or more of your other metal? How much more? Collaborate with your classmates to make sure everyone is making different compositions; compare your samples to your classmates’ samples to better hone in on the compositions of everyone’s samples.

Two students begin melting their alloy test compositions to compare against the unknown sample.
Lab
- Once you decide on possible compositions, weigh each metal you will be using to create the sample. Each sample you make should be about 30–50 g. To calculate the mass of each metal, you will use the following equation to create the binary alloy: %(metal 1) × 50 + %(metal 2) × 50 = 50
- Once you have both metals weighed out, place them in a ceramic crucible.
- Place a mesh square or ceramic triangle on the ring stand with an unlit Bunsen burner placed below.
- Place the crucible on the mesh square and carefully light the Bunsen burner.
- Wait for your metals to melt (about 20–25 minutes), stirring occasionally. Once they are melted, turn off the Bunsen burner and wait for the crucible to cool (about 20 minutes).
- When the crucible is cool enough to touch with your hands, remove it from the ring stand and place it into an ice bath until fully cooled.
- Once it is completely cooled, invert the crucible and firmly hit it against the table. The sample should pop out after a few attempts.
- Once your sample has been removed from the crucible, use a chisel and hammer to break it open. Place pointed side of chisel against center of sample and carefully hit the top of the chisel with a hammer (be careful to avoid fingers!). Sample should break open after a few hits.
- Observe your sample. Does it look like the provided unknown alloy sample? If not, does the sample seem to need more bismuth or more of another metal?
Explain
Once the alloy samples are cooled and cracked open, students compare them to the unknown alloy sample provided at the beginning of lab. Students then complete page 2 of the lab worksheet and record their observations about the sample’s properties and composition. Here, students get a chance to demonstrate their observation skills and some newfound vocabulary (such as cleavage, oxidation, etc.). Students reflect on their findings, and collaborate with other lab groups to determine if the composition of the unknown alloy sample matches the samples they have created.

Comparing the physical properties of the test alloy versus the unknown to determine what revisions should be made.
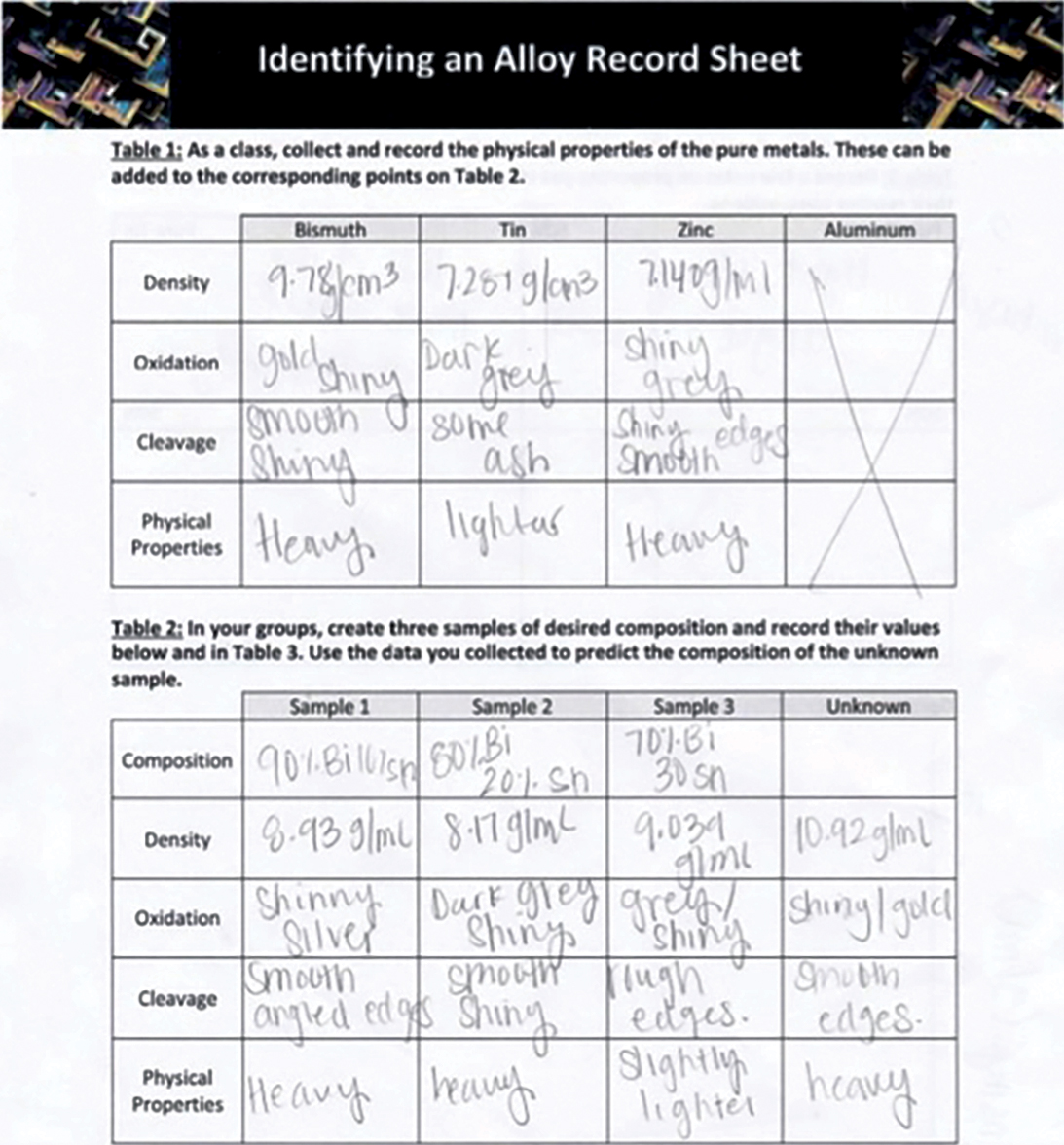
If the entire class worked with the same unknown sample, this can facilitate a larger conversation across groups, allowing students to further demonstrate how to use evidence-based debate and argumentation from data. Using observations gathered in Tables 1–3 of the student worksheet (Figure 6), students are able to determine if their alloy sample matches the unknown sample.

Students produce several compositions in order to extract the most value from each trial.
The three metals students are given to determine which is paired with bismuth all produce distinctly different results. Tin/bismuth composites of a higher tin percentage appear dull and grey. Zinc/bismuth composites develop an oil-slick-like sheen on the surface. (Aluminum/bismuth combinations will not achieve a full melt when using a Bunsen burner.) Once the composition of the unknown sample is determined to be an alloy of bismuth plus zinc or bismuth plus tin, students determine if the unknown sample they verified is suitable for use in structures built for a Martian colony.
Successful synthesis and identification of the teacher- prepared unknown alloy serves as a tether for demonstrated learning, while postlab reflection allows students to weigh the pros and cons of using alloys in construction of future space habitats. Encourage students to think about all the factors at play (safety, weight, fuel costs, etc.) when considering the optimal material for habitat construction.
While aluminum plays the role of red herring in this experiment, it is, of the four metals presented, the ideal base component for use in construction on other worlds. It is lightweight, not brittle, and has a higher melting point. Students should note that compounds with a higher concentration of bismuth were easier to break open with rock hammers.
Consider comparing the density of unknowns to the density of the pure metal samples: How does density affect the ability to mold metals into workable materials or to be launched into space? How does malleability or fracturing make it more or less of a candidate?
Additionally, having already learned how to identify ionic versus covalent bonds, students can have a conversation about the idea of networked solids. While the packing of atoms is likely beyond the rigor of a traditional high school classroom, examples such as diamonds show how impurities in networked solids can result in color differences, while the overall arrangement of atoms can mean the difference between a diamond and graphite. This prompt can be used to have students discuss the differences in their pure starting materials and the homogeneous blends they produce.
Student learning outcomes
As students progress through the design and creation of their sample alloys, our expectations for student outcomes are: Students can design and implement a series of experiments based on an understanding of how chemical properties affect physical features. Students can evaluate data by citing evidence and generate scientific conclusions based on experimental findings connected to real-world phenomena. Students can identify strategies in a small group to delegate a workload of experimentation, and collaborate to achieve a solution using shared results. Students can effectively and safely use laboratory equipment.
Extension
Additional quantities of bismuth can be used to create cubic, hopper-shaped crystals with an oxidation layer coating the crystals in a wide array of colors. Students can pinpoint ideal conditions of crystal growth based on the temperature to which the metal is heated, the rate at which the metal in its liquid state cools, or catalysts used to facilitate growth. Similar to the elementary school project of growing salt crystals on a string submerged in salt water, bismuth can be heated past its low melting point, and as it cools, a “string” of copper or aluminum wire can be submerged in the molten bismuth, then removed before it completely freezes (see Figures 4 and 5). The wire should have cube-shaped crystals of bismuth. Especially impressive crystals can be displayed by the school, given to students to keep, or remelted to perform the extension experiment with another group of students. More in-depth, step-by-step procedures of varying methodologies can be found online (see “On the web”).
Evaluate
Students at both the high school and community college level worked collaboratively across groups to design experiments to uncover the unknown composition of an alloy. Examples of student work are provided in Figure 6. During the lab, most students needed to be explicitly told to calculate the quantitative value for density, or else they were more likely to use relativistic terms such as “most dense” or “least dense.” However, they did use the desired academic vocabulary when organizing their thoughts on physical properties, with one student describing cleavage as “crumbling like a brick” and “heavily oxidized on the sides, bright on the inside.”
We assessed students on their ability to propose a composition based on experimental findings, using evidence obtained not just from their own groups but also from shared data across the class. After organizing their findings and pooling the class data, students predicted the composition of the unknown sample with mixed success. After completing the hands-on lab component, we provided students the following self-assessment questions: How accurately were you able to determine your composition? How would you repeat this process if you had a tertiary alloy (composed of mixed amounts of three metals instead of just two)? Were you able to confidently determine the composition without the use of a density test? Can you identify one scenario where a density test would not be enough by itself? What physical properties of the alloy aided you in determining if composition was within a specified range? Would you build your habitat out of a bismuth-containing alloy? What makes it appealing or what disqualifies it?

By designing alloy compositions as a class, communicating, and sharing data, students are able to identify the unknown sample expediently.
At the close of lab, students complete a postlab survey to communicate their thoughts on the lab. In almost every case—despite having indicated they had little to no knowledge of what an alloy was before the lab began—postlab responses indicated the best way to make the lab more interesting was to include more metal options so they could create more alloys. Two students in particular noted the importance of relative melting times of metals, and how that affected not just alloy formation, but time management within the activity.
Naturally, students are correct: Having more metal options adds an exciting complexity to the activity, and teachers are encouraged to use whatever metals are readily available in their labs. We have provided an editable version of the student worksheet (see “On the web”) to allow for flexibility based on supplies. Teachers are also reminded to check the melting points of metals beforehand.
Conclusion
At the completion of the experiment, students showed a marked rise in interest of alloy analysis. An active learning style—coupled with the excitement of working with molten metals toward the identification of an unknown—allows for students to use logical thinking skills. It also challenges them by allowing loosely restrained, yet safely supervised, experimentation with different combinations and compositions of binary alloys. Educators may also use this experiment as a more in-depth introduction to solid-state chemistry, which is frequently passed over in high school and lower-division college chemistry classrooms.
On the Web
NASA materials and manufacturing
Christina Polcino, Billyjack Jory, Jean Sabety, and Laura Grenot Jones are undergraduate students at Pasadena City College. Jared Ashcroft is a professor in the Natural Sciences Department, Pasadena City College, Pasadena, CA. Brandon Rodriguez (brandon.rodriguez@jpl.nasa.gov) is with NASA Jet Propulsion Laboratory Office of Education, Pasadena, CA.
Chemistry Earth & Space Science Labs High School